This article was written by Dr. Jolene Giacinti (BHSc, DVM, PhD), Wildlife Health Specialist at Environment and Climate Change Canada. Jolene is on the Board of Directors with Veterinarians Without Borders North America/Vétérinaires Sans Frontières Amérique du Nord Canada.
Special thanks to the following individuals for their review and perspectives: DR. ERIN LEONARD AND DR. DANIELLE JULIEN, Veterinary Epidemiologists, Zoonoses Division, Public Health Agency of Canada; DR. HEATHER MCCLINCHEY, Veterinary Consultant, Ministry of Health, Ontario Public Service; DR. DAMIEN JOLY, CEO, Canadian Wildlife Health Cooperative; DR. ANDREA OSBORN, Coordinator, Community for Emerging and Zoonotic Diseases; DR. MURRAY GILLIES, Coordinator, Canadian Animal Health Surveillance System, Animal Health Canada; MICHAEL BROWN, TREVOR THOMPSON, and CYNTHIA PEKARIK, Wildlife Health Unit, Canadian Wildlife Service, Environment and Climate Change Canada; NIAMH BUTLER-CARROLL, PREETI DAVE, and REBECCA MICHELIN, Veterinary Summer Student Interns with Environment and Climate Change Canada.
 Table of Contents:
Table of Contents:
- Introduction
- Understanding the Language
- Low Pathogenicity and Highly Pathogenic Viruses
- Viral Change and Emergence of Clade 2.3.4.4b Viruses
- Dairy Cattle and HPAI A(H5N1): A New Concern
- Current Context in Canada
- Spotlight on Wildlife Surveillance for AIVs
- Everyone Has a Role
- One Health and The Way Forward
Avian influenza viruses (AIVs) are a hot topic right now due to their evolving impacts on wildlife, domestic animals, and humans. As a veterinary epidemiologist with Environment and Climate Change Canada (ECCC), I spend a lot of time in the field sampling wild birds for AIVs and even more time interpreting data and meeting with colleagues and partners from across North America, all to better understand and mitigate the impacts of these viruses (more on that here). Although this topic is far too vast to entirely cover here, I hope this overview answers some common questions and sparks your interest in learning more. The key message is that things are changing quickly – there’s no need for panic, but it’s imperative to be informed and follow best practices.
For those new to this topic, let’s start by decoding some of the complex language and naming conventions.
There are four types of influenza viruses: A, B, C, and D, each with different host species and characteristics. For example, influenza B and C viruses primarily affect humans, whereas influenza D viruses primarily affect cattle. Influenza A viruses have the widest host range but primarily affect birds, humans, or pigs. Avian influenza infections, commonly referred to as bird flu, are caused by influenza A viruses that originate from birds.
Influenza A viruses are classified into subtypes based on two surface proteins: hemagglutinin (H) and neuraminidase (N) (Fig 1). With 18 H subtypes and 11 N subtypes identified, there are many possible subtype combinations (e.g., A(H5N1), A(H7N9)). These surface proteins play a key role in the virus' ability to infect hosts, manifest disease, and spread. During the COVID-19 pandemic, you likely recall hearing about virus ‘spike proteins’ – surface proteins that allow the virus to infect host cells, such as lung tissue for example. The surface proteins of influenza A viruses provide a similar function: H proteins allow the virus to bind to host cells and N proteins allow the virus to release in search of other cells to infect.
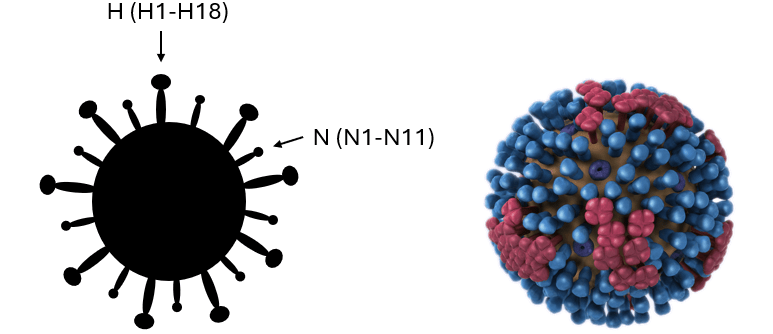
Fig 1. Images of the basic structure of avian influenza viruses demonstrating the H and N surface proteins which dictate virus subtype. The image on the right is a 3D graphical representation obtained from the CDC resource center.
Different versions of the same virus subtype can exist. For example, not all A(H5N1) viruses are the same or behave in the same way. For this reason, additional categorization methods are used.
3. Low Pathogenicity and Highly Pathogenic Viruses
AIVs are classified based on specific genetic characteristics and the severity of the disease they cause in domestic poultry: low pathogenicity avian influenza (LPAI) and high pathogenicity avian influenza (HPAI). Some virus subtypes, such as A(H5N1), can be either LPAI or HPAI.
Most AIVs are of low pathogenicity and cause either no or mild disease in chickens and other domestic poultry. However, some LPAI viruses that are introduced to domestic poultry, particularly H5 and H7 subtypes, can undergo genetic change turning them into HPAI viruses, which can cause severe disease and death in domestic poultry.
For wild birds however, the LPAI/HPAI designation does not indicate the likelihood of disease. Typically, LPAI viruses cause little to no disease in wild birds and can be a natural part of the wild bird virus community. On the other hand, HPAI viruses can result in anything from no signs of illness to severe disease and death, sometimes with large scale and devastating population-level impacts leading to conservation concerns.
Here are a couple of key points to keep in mind:
- "Wild birds" is a broad term often used in the context of AIV but encompasses a huge number of species with vastly different behaviors and likelihoods of infection. In North America and globally, LPAI viruses are most often detected in aquatic bird species like waterfowl and shorebirds, although these species also tend to be the most commonly tested; therefore, the higher rate of detection may be partly due to the increased surveillance efforts in these species.
- HPAI viruses do not naturally occur in wild birds. Historically, LPAI viruses spilled over from wild birds into domestic poultry where they mutated into HPAI before spilling back into wild birds (Fig 2). Wild birds, originally impacted by a domestic poultry virus, are now unfortunately both victims and vectors of A(H5N1) HPAI viruses.
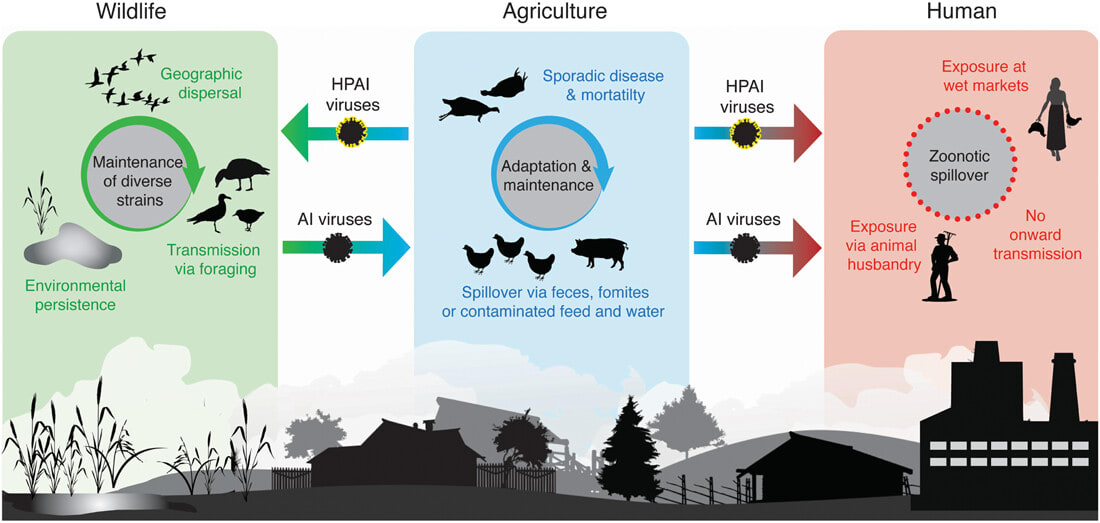
Fig 2. Diagram showing the general ecology of avian influenza viruses. Arrows indicate the usual paths of virus transmission between wildlife, agriculture, and humans, including highly pathogenic viruses. Image obtained from Ramey et al., 2022. Highly pathogenic avian influenza (HPAI): An emerging disease thread in North America.
The LPAI/HPAI designation also does not indicate the likelihood of disease in mammals, including humans. Both LPAI and HPAI virus infections in mammals, including humans, can range from no disease to severe disease and death. Human infections with AIV are rare and almost always result from sustained close contact with infected domestic poultry or heavily contaminated environments. In non-human mammals, ingestion of infected birds and infectious materials (e.g., raw milk), or exposure to contaminated environments are primary risk factors.
4. Viral Change and Emergence of Clade 2.3.4.4b Viruses
Influenza A viruses are notorious for their constant genetic change which occurs in two primary ways. First, they can undergo small changes (mutations) as they replicate in hosts, which can accumulate over time, known as genetic drift. Second, they can experience abrupt, significant changes through reassortment. Reassortment occurs when two different influenza A viruses infect the same cell and exchange gene segments, known as genetic shift. Together, these changes can impact important virus characteristics like how well the virus infects different species, its transmissibility, its susceptibility to antivirals, and its ability to cause disease. Pigs are a frequent conduit for reassortment of influenza A viruses, so preventing transmission to this species is of particular concern and importance. Tracking viral changes helps us to monitor and assess risk, though we are still learning more about the complexities involved.
Because influenza A viruses are constantly evolving, categorizing the different viruses based on their genetic makeup, origin, and behavior helps us better differentiate and understand them. How are viruses named and what can this tell us about them?
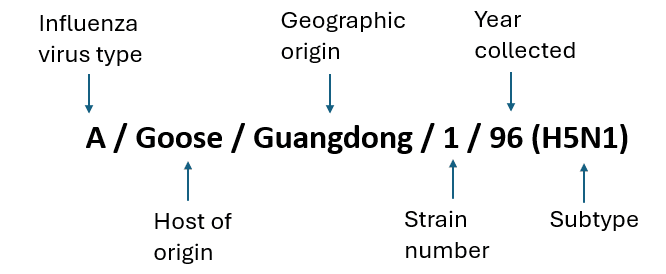
Fig 3. How influenza A viruses are named.
When a sample is collected from an infected human or animal, the virus is described using the host, geographic location, and year of origin, as shown in Fig 3. The group of viruses most relevant to the current outbreak causing concern globally in wildlife, domestic animals, and humans can be traced back to the A/Goose/Guangdong/1/96 virus, originally detected over two decades ago in Asia.
Influenza ‘clades’ are groups of viruses categorized by the similarity of their hemagglutinin gene sequences. Over time, the A/Goose/Guangdong/1/96 virus has undergone genetic change resulting in different clades or groups of viruses – one of which is called clade 2.3.4.4b.
In wild birds, A(H5N1) clade 2.3.4.4b viruses are unprecedented compared to other AIVs in several ways:
- Geographic Range Expansion: They have spread to bird populations across much of the planet, with Oceania being the only region currently free of A(H5N1) clade 2.3.4.4b viral detections in birds or mammals (although there have been recent poultry H7 detections and a human non-2.3.4.4b A(H5N1) detection in Australia). More information on the global distribution of AIVs is available on the WOAH or FAO websites.
- Host Range Expansion: Detections in at least 500 species of wild birds, with over half of these being newly reported since 2021. Additionally, over 60 mammalian species have been affected, many of which were also newly reported since the emergence of clade 2.3.4.4b viruses. More information and global updates regarding affected species are available on the FAO website.
- Maintenance in Asymptomatic Wild Birds: Over time, A(H5N1) clade 2.3.4.4b viruses have adapted to some species of wild birds, primarily dabbling ducks, in which they can cause little to no disease thereby facilitating widespread geographic expansion in these migratory species.
- Unprecedented Wildlife Mortality: The impact on wildlife has been severe across the globe, with some species (for instance, the Northern Gannet) experiencing significant population declines worldwide due to A(H5N1) clade 2.3.4.4b viruses (Fig. 4). More information on AIV related mass mortalities in wild birds in eastern Canada is available as a preprint publication.
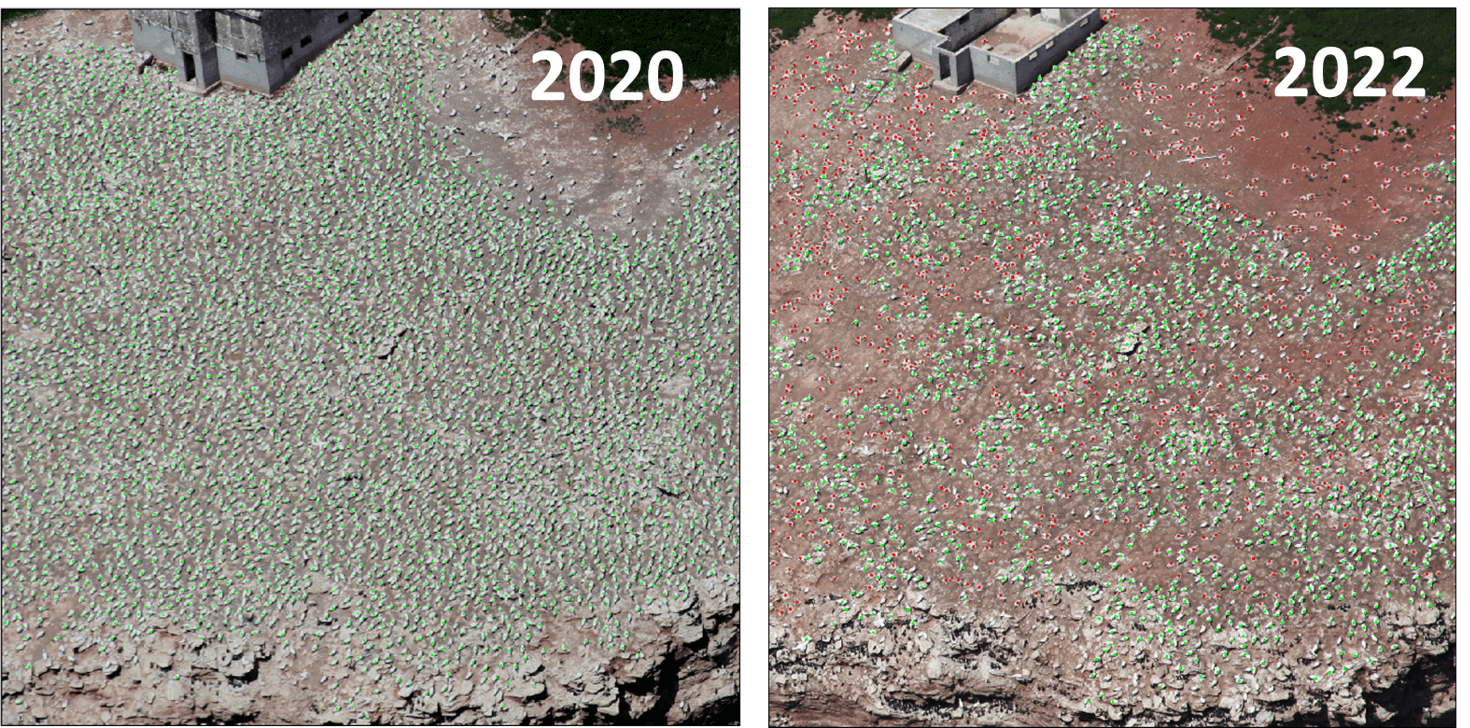
Fig 4. Dramatic decrease in the Northern Gannet colony at Rocher aux Oiseaux (Bird Rocks), Quebec between 2020 and 2022. Green dots represent active nesting sites, while red dots indicate dead birds. A total of 5,119 dead birds and a 58% decline in active nests were reported in 2022 as a result of A(H5N1). (Photo Credit: JF Rail, ECCC)
5. Dairy Cattle and HPAI A(H5N1): A New Concern
The impact of A(H5N1) clade 2.3.4.4b viruses has been unprecedented in many sectors, including detections in dairy cattle and unpasteurized milk in the United States in 2024, that have sparked international concern. Cattle have not historically been considered important hosts for influenza A viruses.
As of July 16th, 2024, the United States Department of Agriculture (USDA) reports 158 confirmed cases of A(H5N1) clade 2.3.4.4b virus in livestock herds across 13 states, with 52 cases emerging in the previous 30 days, particularly in Colorado. For the latest updates and more detailed information on cases in livestock, the USDA dashboard and reports can be found here.
Cows affected by A(H5N1) clade 2.3.4.4b virus show signs like decreased milk production, thicker consistency milk resembling colostrum, and reduced feed consumption and rumen motility. Other signs include fever, lethargy, and nasal discharge. Most affected cows appear to recover after a period of illness. Raw milk from these infected cows has been found to contain high levels of active virus and is believed to be one of the primary sources of viral transmission. However, pasteurized milk remains safe to consume as pasteurization has been found to be effective against this group of viruses.
It is suspected that the outbreak in dairy cattle originated from a single introduction from a wild bird, with subsequent spread attributed to dairy cattle movement, contaminated equipment, and shared employees between farms. There have been reports of sick and dead wild birds, domestic cats, and poultry on or connected to affected dairy farms, as well as detections of the virus in a species of mouse known to occur on or near both dairy and poultry operations.
On April 1st, 2024, the US Centers for Disease Control and Prevention (CDC) confirmed the first human avian influenza A(H5N1) infection in a person exposed to dairy cattle presumed to be infected with A(H5N1) viruses. Since then, there have been additional sporadic human cases among workers on dairy farms where cows tested positive for A(H5N1) virus. Human cases have also been detected in the US among workers on poultry farms where birds tested positive for A(H5N1) virus. The range of symptoms has involved eye symptoms, such as eye redness (consistent with conjunctivitis) and/or mild respiratory symptoms (e.g., coughing); cases were given antiviral treatment and resolved.
Wildlife: In Canada, A(H5N1) clade 2.3.4.4b viruses have been detected in wildlife across all provinces and territories. Thankfully, the number of infections detected in wild birds and mammals, as well as mortality events, was lower in 2023 compared to 2022. While 2024 is still unfolding, the numbers remain similarly low, so far. For up-to-date information about detections in wildlife in Canada visit the dashboard. For more information about AIV in wild birds including clinical signs visit ECCC’s webpage.
Domestic animals: Cases of A(H5N1) clade 2.3.4.4b virus in domestic poultry also saw a decrease in 2023 compared to 2022; as of July 17th, 2024, the last reported detected in domestic poultry in Canada was on April 10, 2024. As of July 17th, 2024, there have been no detections in dairy cows or pasteurized milk in Canada; 911 retail milk samples have been tested to date by the Canadian Food Inspection Agency (CFIA) for evidence of A(H5N1) viral fragments; to date all samples have been negative. Tightened import controls, pre-movement testing of lactating dairy cattle, and the relatively low number of cattle imported from the US (plus probably a bit of luck) seem to be working in our favour. There have also been cases reported in feral cats and a domestic dog. Primary risk factors include hunting or scavenging of infected birds (and in the US, cats with access to raw milk from infected cows).
Humans: Regarding the current A(H5N1) clade 2.3.4.4b outbreak in animals, as of July 17th, 2024, there have been no human cases detected in Canada. However, the increased presence of these viruses in wild birds, domestic poultry, and some mammals may increase opportunities for human exposure. This could lead to more sporadic human infections, even though the overall risk of avian influenza infection to the general public in Canada currently remains low. Those who have close contact with infected domestic poultry, livestock, and wildlife, are at an increased risk.
In North America, there have been no reported cases of avian influenza infection in humans resulting from exposure to or consumption of wild mammals, wild birds, or eggs. However, these viruses continue to be detected in species that are commonly harvested, such as dabbling ducks and wild mammals that commonly ingest wild birds (e.g., foxes). This ongoing presence underscores the importance of continuous monitoring and preventive measures to mitigate potential risks to human health.
Pasteurized cow's milk and milk products in Canada remain safe to consume. Milk from dairy cows in Canada must be pasteurized before sale. While the sale of raw milk is not legal in Canada, it's worth noting that there are reports of raw milk being accessed through informal markets and raw milk is currently legal in around 30 U.S. states, including some that share a border with Canada.
More about what we are doing in Canada: Canada's approach to managing A(H5N1) involves a collaboration of federal, provincial, territorial, non-governmental, and Indigenous authorities, industry stakeholders, Animal Health Canada, academic and international partners, across human, animal, and environmental sectors. Measures range from surveillance, risk assessments, enhanced import controls, and biosecurity guidance, to preparedness and proactive measures. More information can be found here.
7. Spotlight on Wildlife Surveillance for AIVs
Wildlife surveillance is a cornerstone of Canada’s response. Canada's Interagency Surveillance Program for Avian Influenza Viruses in Wildlife, which has been ongoing since 2005, has been crucial in tracking the introduction, spread, and evolution of AIVs. This program, a collaborative effort involving federal, provincial, territorial, Indigenous, and academic partners, comprises two main components:
- Sick and Dead Wildlife Surveillance: This involves testing sick or dead wild birds and mammals reported to the Canadian Wildlife Health Cooperative or relevant provincial/territorial wildlife authority. These efforts provide critical data on AIV-related mortality events and have identified a wide range of susceptible species.
- Live and Harvested Wild Bird Surveillance: Samples are collected from live caught or hunter-harvested wild birds. Swabs (Fig 5) help detect the virus and track its evolution, while blood samples (Fig 5) provide insights into exposure and survival, especially in species of conservation concern, including Species at Risk. Apparently healthy dabbling ducks are the primary group that have been found positive with A(H5N1) clade 2.3.4.4b viruses. Across multiple species we see an increase in antibody presence from 2022 to 2023, suggesting increased exposure and survival following infection with these viruses in many species.
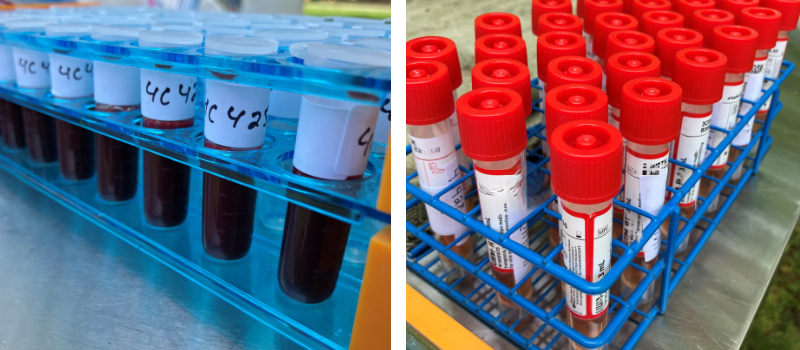
Fig 5. On the left are blood samples collected from live birds for avian influenza antibody testing. On the right are swabs collected from live birds for avian influenza virus testing. The birds are caught, sampled, and released unharmed as part of Canada’s Interagency Surveillance Program for Avian Influenza Viruses in Wildlife. (Photo credit: M. Brown)
These efforts aim to optimize virus detection, track changes in the virus, and understand exposure and survivability among wildlife populations. To learn more, you can explore the AIV dashboard. A summary of surveillance activities and results is also available as an open access publication.
Decreasing the risks associated with AIVs is a collective effort, and everyone has a role to play.
Veterinarians and Producers:
- You are on the front lines of detection in Canada’s domestic and wild species.
- Biosecurity remains the most effective strategy to mitigate risks on domestic premises. Advocate for and implement best practices for biosecurity to reduce potential for both animal and human infections. There are numerous resources available, including: CFIA National Avian On-Farm Biosecurity Standard & Producer Guide; DFC and CFIA National Standard for Biosecurity on Dairy Farms; AABP Dairy Biosecurity Recommendations; AHC Biosecurity Infographic for Dairy Farmers.
- Collaborate within your networks and with relevant authorities on infection prevention and management.
- Include A(H5N1) as a differential diagnosis, even for non-avian species, that are showing relevant clinical signs, or that are found sick or dead on or near affected premises, and/or healthy animals with a history of exposure to infected animals or contaminated animal environments or products.
- Report suspected cases – remember HPAI is a federally reportable disease in any species. If you suspect HPAI, report it to your local CFIA district office. HPAI is also reportable in some provinces and territories.
- Stay up to date on evidence-based information and act as a resource among your clients and community.
Wildlife Rehabilitators:
- You are on the front lines of detecting sick and dead wildlife – report cases to the Canadian Wildlife Health Cooperative or relevant provincial/territorial wildlife authority.
- For more information on wildlife handling guidelines visit the Public Health Agency of Canada’s (PHAC) webpage.
- Implement best practices for biosecurity and quarantine.
- Facilitate the submission of samples for testing using approved and robust methods.
- Consult credible resources to stay up to date and ask questions!
Everyone:
- Avoid handling wildlife where possible, especially if they appear sick or are found dead. Report sick and dead wildlife to the Canadian Wildlife Health Cooperative or relevant provincial/territorial wildlife authority.
- For more information on wildlife handling guidelines visit the PHAC webpage.
- Hunters and trappers are recommended to take precautions while handling wild animals and follow other safe food handling guidelines which can be found here.
- Pet owners are advised to keep pets supervised while outdoors and to keep dogs on a leash during walks. More information on steps you can take to decrease risk for your pets can be found here.
- Don’t consume (or feed your pets) undercooked meat, eggs, or unpasteurized dairy products.
- Wear appropriate personal protective equipment if closely interacting with sick or dead birds or other animals.
- Consult your local public health authority to get tested for influenza if you have been exposed to infected animals or contaminated environments or products in the past 10 days and you have signs of flu.
- Get your seasonal influenza vaccine every year to prevent co-infection with multiple influenza viruses.
- Consult credible resources, such as those listed throughout this document, to stay up to date and ask questions!
9. One Health and The Way Forward
As avian influenza continues to evolve, so must our strategies. Managing avian influenza effectively requires recognizing the interconnectedness of human, animal, and environmental health. We need bold and creative ideas to drive change!
We can all help support One Health (Fig. 6) by advocating for the protection of wetland habitats and the separation of domestic poultry and other livestock facilities from these habitats. This not only helps prevent spillover and spillback of AIVs between wild and domestic birds and other animals, but also supports biodiversity and conservation for these important and dynamic species.
Working collaboratively across sectors, supported by rigorous scientific research, routine surveillance, and robust biosecurity measures, remains our best defense against this and future emerging pathogens.
Fig 6. Infographic depicting the One Health concept developed by the One Health High Level Expert Panel.





